Publication
Research Article
International Journal of MS Care
Reliability and Validity of a Danish Version of the Multiple Sclerosis Neuropsychological Screening Questionnaire
Author(s):
CME/CNE Information
Activity Available Online:
To access the article, post-test, and evaluation online, go to http://www.cmscscholar.org.
Target Audience:
The target audience for this activity is physicians, physician assistants, nursing professionals, and other health-care providers involved in the management of patients with multiple sclerosis (MS).
Learning Objectives:
1) Describe cognitive impairment in MS and understand differences between patient-reported outcome scales and neuropsychological testing.
2) Understand the design of validation studies and be aware that results from translated and unvalidated patient-reported outcome scales may result in bias.
Accreditation Statement:

In support of improving patient care, this activity has been planned and implemented by the Consortium of Multiple Sclerosis Centers (CMSC) and Delaware Media Group. CMSC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Credit
The CMSC designates this journal-based activity for a maximum of .75 AMA PRA Category 1 Credit(s) ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Nurse Credit
The CMSC designates this enduring material for .75 contact hours.
Disclosures:
Editor in Chief of the International Journal of MS Care (IJMSC), has served as Physician Planner for this activity. He has received royalties from Springer Publishing; has received consulting fees from Ipsen; and has performed contracted research for Biogen, Adamas Pharmaceuticals, and Acorda Therapeutics.Francois Bethoux, MD,
has served as reviewer for this activity. She has disclosed no relevant financial relationships.Laurie Scudder, DNP, NP,
has received honoraria from lectures at a Biogen symposium; has performed contracted research as principal investigator of a phase 4 trial with support from Biogen; has served on the scientific advisory boards of Novartis, Biogen, Teva, Merck, and Roche; and has received travel funding or speaker honoraria from Biogen, Teva, and Novartis.Tobias Sejbæk, MD,
has received honoraria from lectures at a symposium organized by Biogen Denmark.Morten Blaabjerg, MD, PhD,
has disclosed no relevant financial relationships.Pippi Sprogøe, MSc,
has performed contracted research as principal investigator of phase 3 and 4 studies by Biogen, Roche, Novartis, and Genzyme.Mads Ravnborg, MD, DMsc,
The peer reviewers for the IJMSC have disclosed no relevant financial relationships.
The staff at the IJMSC, CMSC, and Delaware Media Group who are in a position to influence content have disclosed no relevant financial relationships.
Note: Disclosures listed for authors are those applicable at the time of their work on this project and within the previous 12 months.
Method of Participation:
Release Date: February 1, 2018
Valid for Credit Through: February 1, 2019
In order to receive CME/CNE credit, participants must:
1) Review the continuing education information, including learning objectives and author disclosures.
2) Study the educational content.
3) Complete the post-test and evaluation, which are available at http://www.cmscscholar.org.
Statements of Credit are awarded upon successful completion of the post-test with a passing score of >70% and the evaluation.
There is no fee to participate in this activity.
Disclosure of Unlabeled Use:
This educational activity may contain discussion of published and/or investigational uses of agents that are not approved by the FDA. CMSC and Delaware Media Group do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of CMSC or Delaware Media Group.
Disclaimer:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any medications, diagnostic procedures, or treatments discussed in this publication should not be used by clinicians or other health-care professionals without first evaluating their patients' conditions, considering possible contraindications or risks, reviewing any applicable manufacturer's product information, and comparing any therapeutic approach with the recommendations of other authorities.
Abstract
Background:
More than half of all patients with multiple sclerosis (MS) acquire cognitive impairment as part of their disease progression. Because cognitive dysfunction adds substantially to disability and coping strategies, a cost-effective screening tool is needed for cognitive impairment. The Multiple Sclerosis Neuropsychological Screening Questionnaire (MSNQ) has previously shown good validity in American, Argentinean, and Dutch MS cohorts. We sought to test reliability and validity of a Danish translation of the MSNQ compared with formal neuropsychological testing, and measures of depression and disability, and to compare self-reported cognition with Symbol Digit Modalities Test (SDMT) results.
Methods:
Of 126 patients with MS and their informants tested with the MSNQ, 77 also underwent formal neuropsychological testing. All patients were tested with the SDMT and assessed clinically using the Expanded Disability Status Scale and MS Impairment Scale.
Results:
The test-retest reliability of the MSNQ-P was significant (R2 = 0.79, P < .0001). R2 of informants (MSNQ-I) and patients (MSNQ-P) was much lower (R2 = 0.22, P < .0001). Compared with formal neuropsychological testing, the MSNQ-P and MSNQ-I performed poorly, with no correlation to individual neuropsychological tests, combined neuropsychological tests, or disability scores (Expanded Disability Status Scale and MS Impairment Scale). Depression/anxiety (Beck Depression Inventory) showed a weak linear relationship (R2 = 0.25, P < .0001), suggesting that the MSNQ-P measures these items more than the cognitive abilities of the patients.
Conclusions:
This study does not support use of the MSNQ as a sensitive or valid screening tool for cognitive impairment in Danish patients with MS.
Cognitive impairment is a common problem in multiple sclerosis (MS), occurring in approximately 50% of patients. It typically involves memory, information-processing speed, learning, and executive function and, thereby, affects activities of daily living and quality of life.1 2 Moreover, cognitive impairment is often the primary cause of unemployment, reported in 70% to 80% of all patients with MS.1 3 4 Cognitive impairment may prove to be the only parameter in a few patients with MS and especially in patients with MS treated with immunomodulatory drugs that can induce progressive multifocal leukoencephalopathy. Cognitive screening is, thus, incredibly valuable as a means of assessment and as a guide for treatment.5 6 Considering the prevalence and morbidity, there is a need for cost-effective screening tools for cognitive impairment in MS.
The best-validated fast screening test in patients with MS is the Symbol Digit Modalities Test (SDMT). This test is easy to administer and correlates well with cognitive impairment as measured by other standardized neuropsychological batteries assessing multiple functions and brain atrophy.7 The SDMT, however, addresses only a fraction of the cognitive functions, such as process speed and visuospatial and working memory.8 9
The Multiple Sclerosis Neuropsychological Screening Questionnaire (MSNQ) is a brief patient-reported outcome scale by either the patient (MSNQ-P) or the informant (MSNQ-I) and is easy to administer. It has previously been shown to have acceptable reproducibility and to provide valid assessment of cognitive dysfunction in American, Argentinean, and Dutch populations. In contrast to the SDMT, the MSNQ can provide information about self- and informant-perceived cognitive dysfunction.10–13
The objective of this study was to validate a Danish translation of the MSNQ compared with formal neuropsychological testing and with the SDMT and measures of depression and disability.
Methods
Ethics
All the procedures were performed according to the Declaration of Helsinki and with permission from the Danish Data Protection Agency (14/8330).
Translation of MSNQ
The MSNQ was translated by a bilingual translator from English to Danish and then was translated from Danish to English by another translator to correct for any linguistic corrections or oversights.
Study Population
We studied 126 patients diagnosed as having MS in the Department of Neurology, Odense University Hospital (Odense, Denmark), over 7 consecutive years (January 1, 2000, through December 31, 2006). The inclusion criteria were 1) a diagnosis of MS according to the McDonald criteria,14 2) an informant with face-to-face contact with the patient three or more times a week, 3) age older than 18 years, 4) Danish as the first language, and 5) informed consent. The exclusion criteria were 1) neurologic deficits not related to MS, 2) a history of developmental disorders or other learning disabilities, 3) previous or present psychiatric disease that is unlikely to be part of the patient's MS, 4) alcohol or drug abuse, and 5) corticosteroid treatment in the 4 weeks before evaluation. Informants were selected by the following criteria: closest family or friend with whom the patient lives or has at least three weekly contacts, in the following prioritized rank: spouse, father/mother, daughter/son, friend/closest family.
Application of MSNQ, SDMT, and Neuropsychological Testing
Patients and their respective informants were carefully given standardized instructions on how to fill out the MSNQ10 and the Beck Depression Inventory (BDI)15 during each contact. Patients completed questionnaires in the department, and a neurologist (M.B. or T.S.) assessed clinical deficits by means of the MS Impairment Scale (MSIS),16 17 the Expanded Disability Status Scale (EDSS),18 and the SDMT.19 20
Informants completed the MSNQ-I during the visit or within a few weeks. The first 77 patients examined were chosen for neuropsychological testing, which occurred a few weeks after contact with the neurologist. The test battery comprised the following tests: the Rey Auditory Verbal Learning Test, the Trail Making Test B, the Wisconsin Card Sorting Test, the Boston Naming Test, and Digit-Symbol Coding. A z score of the combined neuropsychological tests was calculated from the test results, and a z score less than −1.5 SD was used as the cutoff point to diagnose true cognitive impairment.
Based on earlier studies, a cutoff score of 26 points or greater on the MSNQ-I and the MSNQ-P was chosen as a sign of cognitive impairment.10 12 13 21 22 Forty-four patients were retested (Figure 1) to investigate the test-retest variability of the MSNQ-I and the MSNQ-P.
Study flowchart
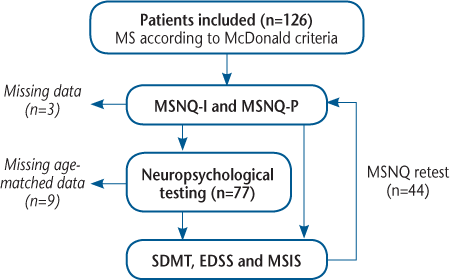
Statistical Methods
Z scores were based on sex- and age-matched controls. Z scores could not be calculated for nine patients due to either missing age-matched normal values (patients >60 years old) or incomplete neuropsychological testing. Three informants did not return the MSNQ-I (Figure 1). Statistical analyses were performed using GraphPad PRISM 7 (GraphPad Software Inc, La Jolla, CA). Because data were not distributed normally, nonparametric statistics were used.
Results
Demographics and Comparison of Subgroups
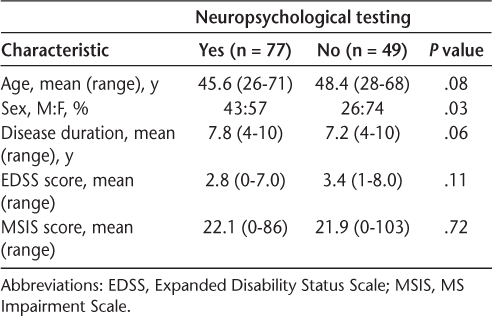
Comparing patients who underwent neuropsychological testing (n = 77) with those who did not (n = 49), we found no differences in demographics in relation to age, disease duration, and disability as measured by the EDSS and the MSIS. There were also more men in the neuropsychological group (43%) compared with in the group that was not tested by a neuropsychologist (26%) (P < .05) (Table 1).
Baseline demographic characteristics of the 126 study participants
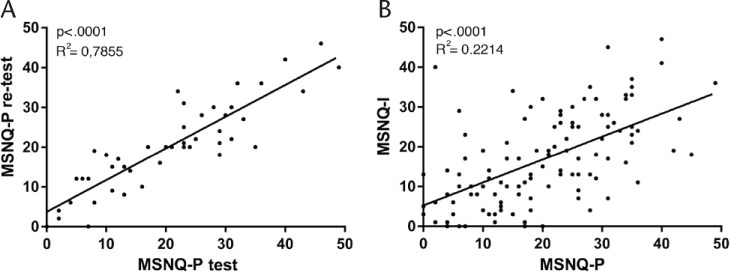
Test-Retest of MSNQ-P and Correlation Between MSNQ-I and MSNQ-P
The squared test-retest correlation of the MSNQ-P was R 2 = 0.79 (P < .0001) (Figure 2A) and the squared correlation of the informants (MSNQ-I) and patients (MSNQ-P) was low but significant (R 2 = 0.22, P < .0001) (Figure 2B).
Linear fit regression of Multiple Sclerosis Neuropsychological Screening Questionnaire–Patient (MSNQ-P) and MSNQ-Informant (MSNQ-I)
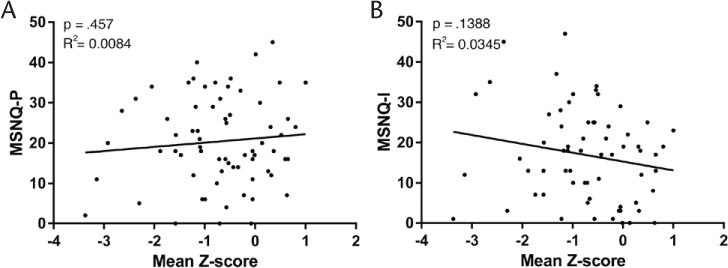
Squared Correlation Between MSNQ and Neuropsychological Tests and BDI
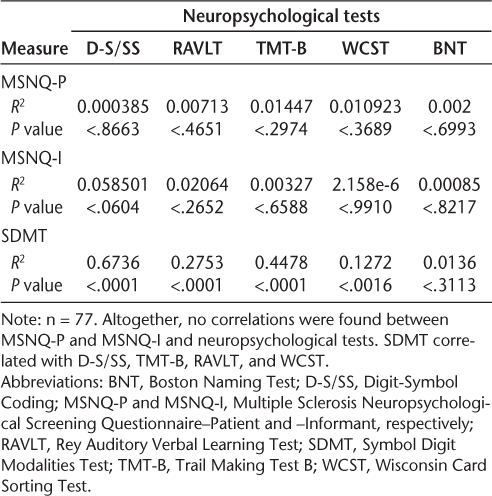
When correlating the MSNQ-I or the MSNQ-P (greater than the cutoff score of 26 points) with the z score of the complete neuropsychological test battery, we found no significant squared correlation with either scale (MSNQ-P: R 2 = 0.0084, P = .457; MSNQ-I: R 2 = 0.0345, P = .1388) (Figure 3). Neither did the MSNQ-I and the MSNQ-P correlate with the individual neuropsychological tests (Table 2). We found low sensitivity and specificity of the MSNQ-P (21.4% and 76%, respectively) and the MSNQ-I (33% and 65.5%, respectively). Both scales did, however, correlate to BDI scores (MSNQ-P: R 2 = 0.25, P < .0001; MSNQ-I: R 2 = 0.197, P < .0001 [data not shown]).
Linear fit regression of Multiple Sclerosis Neuropsychological Screening Questionnaire–Patient (MSNQ-P) or MSNQ-Informant (MSNQ-I) and neuropsychological test battery
Correlations between MSNQ-P, MSNQ-I, and SDMT and individual neuropsychological tests
Squared Correlation Between MSNQ and Disability Scores
To investigate whether there is a correlation between cognitive dysfunction measured by the MSNQ and disability, we compared MSNQ-I with EDSS and MSIS scores. We found no squared correlation to either objective disability measure (EDSS: R 2 = 0.0036, P = .5; MSIS: R 2 = 0.0001, P = .89 [data not shown]).
Correlation of SDMT and Neuropsychological Testing
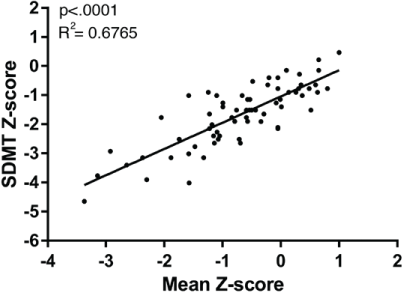
As a positive control for bedside neuropsychological testing in patients with MS, we also tested the squared correlation between the well-established SDMT and the total neuropsychological test panel. As expected, this squared correlation was highly significant (R 2 = 0.68, P < .0001) (Figure 4). When correlated to the individual neuropsychological tests, we found the best squared correlations to Digit-Symbol Coding and the Trail Making Test B. The SDMT also correlated to the Rey Auditory Verbal Learning Test and the Wisconsin Card Sorting Test but not to the Boston Naming Test (Table 2).
Linear fit regression of Symbol Digit Modalities Test (SDMT) and neuropsychological test battery
Discussion
The results of the present study show that a Danish translation of the MSNQ, despite good test-retest reliability, has no statistically significant correlation to cognitive impairment found on standard neuropsychological testing. This is in contrast to validation studies in other languages.12 13 21 22 When using the now well-established SDMT scale, we did, however, find a highly significant correlation with the neuropsychological test battery, demonstrating the superiority of this scale in establishing the presence of cognitive impairment in MS.23–26 Several factors may explain the low sensitivity and specificity of the MSNQ in the present study. First, we used an MSNQ cutoff score greater than 26, which is the cutoff score used in the original validation.10 Other studies have used different cutoff values to achieve higher sensitivity and specificity, and the optimal cutoff score seems to change from study to study.13 22 27 Using other cutoff points did not increase the sensitivity or specificity in the present population.
Second, compared with most of the earlier MSNQ validation studies performed,13 22 27 the present cohort demonstrated a shorter disease duration (mean, 7.8 years), a lower EDSS score (mean, 2.8), and fewer cognitively impaired patients (13 of 77). A screening tool for cognitive impairment should, however, be relevant also in younger MS cohorts with shorter disease durations, and, when using the SDMT in the present cohort, we did find very high sensitivity compared with neuropsychological testing.
Sonder et al27 demonstrated lower loadings regarding how individual questions are weighted and affect the MSNQ score. Different study populations and cultural differences regarding the content of the questions should also be considered in future studies. Unfortunately, this study was not designed to test for such differences.
Finally, it should be considered whether the MSNQ actually does measure cognitive impairment. Strober et al28 recently reported that self-assessed measures of cognition do not correlate with systematic neuropsychological testing but rather with quality of life and behavioral data. We found that the MSNQ-I and the MSNQ-P correlated significantly with BDI scores. Other studies have also suggested that the MSNQ is influenced by psychosocial variables, such as anxiety, rather than by objective status.13 29 30 The impact of psychosocial variables could be the explanation for the subpopulation of patients who reported high impact on self-experienced cognitive impairment but had normal results on standard neuropsychological testing.
An opposite subgroup with low MSNQ-I and MSNQ-P scores had severe cognitive impairment when tested with formal neuropsychological tests. This might be explained by a coping strategy of the patients and informants to undervalue cognitive impairment and, therefore, report low MSNQ-P and MSNQ-I scores in self-perceived cognitive impairment. Another explanation for low MSNQ-P scores and high cognitive impairment could also be that severely affected patients do not perceive the dementia. Further studies are needed to clarify these issues of self-assessed measures of cognition.
In conclusion, this study does not support use of the MSNQ-P or the MSNQ-I as a sensitive or valid screening tool for cognitive impairment in Danish patients with MS. In contrast, this study found the SDMT to be more reliable owing to higher sensitivity and specificity. Further studies are needed to assess the effect of differences in study populations, choice of cutoff values, and cultural and psychosocial impact.
PRACTICE POINTS
We studied the validity and reliability of the Multiple Sclerosis Neuropsychological Screening Questionnaire (MSNQ), a screening tool for cognitive impairment, in 126 Danish patients and their informants.
Test-retest reliability of the MSNQ-Patient (MSNQ-P) was assessed on a subsample of 44 patients and was found to be high. There was a high correlation between the MSNQ-P and the MSNQ-Informant (MSNQ-I).
The Danish versions of the MSNQ-P and the MSNQ-I showed no correlation with formal neuropsychological testing. In contrast, they demonstrated significant (albeit low) correlation with depression, suggesting correlation of the Danish MSNQ-P and MSNQ-I with behavioral outcomes rather than with neuropsychological measures.
Acknowledgments:
We thank the Desirée and Niels Yde Foundation for its hospitality and Christian Schoubye for bridging the language gap.
References
Glanz BI, Healy BC, Rintell DJ, Jaffin SK, Bakshi R, Weiner HL. The association between cognitive impairment and quality of life in patients with early multiple sclerosis. J Neurol Sci. 2010;290:75–79.
Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis, I: frequency, patterns, and prediction. Neurology. 1991;41:685–691.
Baumstarck-Barrau K, Simeoni MC, Reuter F, et al. Cognitive function and quality of life in multiple sclerosis patients: a cross-sectional study. BMC Neurol. 2011;11:17.
Rao SM, Leo GJ, Ellington L, Nauertz T, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis, II: impact on employment and social functioning. Neurology. 1991;41:692–696.
Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–446.
Hellwig K, Gold R. Progressive multifocal leukoencephalopathy and natalizumab. J Neurol. 2011;258:1920–1928.
Vollmer T, Huynh L, Kelley C, et al. Relationship between brain volume loss and cognitive outcomes among patients with multiple sclerosis: a systematic literature review. Neurol Sci. 2016;37:165–179.
McCaffrey RJ, Krahula MM, Heimberg RG, Keller KE, Purcell MJ. A comparison of the trail making test, symbol digit modalities test, and the Hooper Visual Organization Test in an inpatient substance abuse population. Arch Clin Neuropsychol. 1988;3:181–187.
Benedict RH, Fischer JS, Archibald CJ, et al. Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol. 2002;16:381–397.
Benedict RH, Munschauer F, Linn R, et al. Screening for multiple sclerosis cognitive impairment using a self-administered 15-item questionnaire. Mult Scler. 2003;9:95–101.
Benedict RH, Weinstock-Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R. Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol. 2004;61:226–230.
Benedict RH, Zivadinov R. Reliability and validity of neuropsychological screening and assessment strategies in MS. J Neurol. 2007;254(suppl 2):II22–II25.
O'Brien A, Gaudino-Goering E, Shawaryn M, Komaroff E, Moore NB, DeLuca J. Relationship of the Multiple Sclerosis Neuropsychological Questionnaire (MSNQ) to functional, emotional, and neuropsychological outcomes. Arch Clin Neuropsychol. 2007;22:933–948.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50:121–127.
Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597.
Ravnborg M, Blinkenberg M, Sellebjerg F, Ballegaard M, Larsen SH, Sorensen PS. Responsiveness of the Multiple Sclerosis Impairment Scale in comparison with the Expanded Disability Status Scale. Mult Scler. 2005;11:81–84.
Ravnborg M, Gronbech-Jensen M, Jonsson A. The MS Impairment Scale: a pragmatic approach to the assessment of impairment in patients with multiple sclerosis. Mult Scler. 1997;3:31–42.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444–1452.
Smith A. Symbol Digit Modalities Test: Manual. Los Angeles, CA: Western Psychological Services; 1982.
Watson CG, Gasser B, Schaefer A, Buranen C, Wold J. Separation of brain-damaged from psychiatric patients with ability and personality measures. J Clin Psychol. 1981;37:347–353.
Benedict RH, Cox D, Thompson LL, Foley F, Weinstock-Guttman B, Munschauer F. Reliable screening for neuropsychological impairment in multiple sclerosis. Mult Scler. 2004;10:675–678.
Vanotti S, Benedict RH, Acion L, Caceres F. Validation of the Multiple Sclerosis Neuropsychological Screening Questionnaire in Argentina. Mult Scler. 2009;15:244–250.
Benedict RH, Duquin JA, Jurgensen S, et al. Repeated assessment of neuropsychological deficits in multiple sclerosis using the Symbol Digit Modalities Test and the MS Neuropsychological Screening Questionnaire. Mult Scler. 2008;14:940–946.
Morrow SA, O'Connor PW, Polman CH, et al. Evaluation of the symbol digit modalities test (SDMT) and MS neuropsychological screening questionnaire (MSNQ) in natalizumab-treated MS patients over 48 weeks. Mult Scler. 2010;16:1385–1392.
Sonder JM, Burggraaff J, Knol DL, Polman CH, Uitdehaag BM. Comparing long-term results of PASAT and SDMT scores in relation to neuropsychological testing in multiple sclerosis. Mult Scler. 2014;20:481–488.
Van Schependom J, D'Hooghe MB, Cleynhens K, et al. The Symbol Digit Modalities Test as sentinel test for cognitive impairment in multiple sclerosis. Eur J Neurol. 2014;21:1219–1225.
Sonder JM, Mokkink LB, van der Linden FA, Polman CH, Uitdehaag BM. Validation and interpretation of the Dutch version of the Multiple Sclerosis Neuropsychological Screening Questionnaire. J Neurol Sci. 2012;320:91–96.
Strober LB, Binder A, Nikelshpur OM, Chiaravalloti N, DeLuca J. The Perceived Deficits Questionnaire: perception, deficit, or distress? Int J MS Care. 2016;18:183–190.
Akbar N, Honarmand K, Feinstein A. Self-assessment of cognition in multiple sclerosis: the role of personality and anxiety. Cogn Behav Neurol. 2011;24:115–121.
Langdon DW, Benedict RH, Wicklein EM, Beckmann K, Fredrikson S. Reports of patients and relatives from the CogniCIS study about cognition in clinically isolated syndrome: what are our patients telling us? Eur Neurol. 2013;69:346–351.







