Publication
Research Article
International Journal of MS Care
Relationship Between Disease-Modifying Therapy and Depression in Multiple Sclerosis
Author(s):
Many prescribers of disease-modifying therapies (DMTs) for multiple sclerosis (MS) believe that interferon beta (IFNβ) is more likely than glatiramer acetate (GA) to increase depression during the course of MS treatment. Therefore, newly diagnosed patients with a history of depression are often placed on GA therapy from the onset of MS treatment. The aim of this study was to examine the relationship between DMT type and depression among patients with relapsing-remitting MS (RRMS). Patients with RRMS who were examined from 2000 to 2007 and who remained on a single course of therapy (either an IFNβ or GA) were included in a retrospective review of medical records. Patients were asked to complete the Beck Depression Inventory (BDI) at treatment initiation and every 6 months thereafter for up to 4 years. Only patients who had completed a BDI within 6 weeks of starting their DMT were included in the analysis. No significant differences in mean change in BDI score were observed from baseline to 48 months between the IFNβ and GA subgroups. Additionally, no significant differences in mean BDI score change were observed between antidepressant-treated and non–antidepressant-treated patients within the IFNβ or GA subgroup. Neither IFNβ nor GA therapy appears to exacerbate depressive symptoms in patients with RRMS who remain on their initial therapy.
Depression is a condition experienced by a large number of patients living with multiple sclerosis (MS), with lifetime prevalence estimates ranging from 40% to 60%.1 2 At any point, it is estimated that 15% to 30% of patients with MS are depressed, and an absence of depression during disease progression and treatment does not exclude the possibility that depression may occur during different stages of disease.2 The seriousness of depression in MS is illustrated by the fact that more than 40% of depressed patients with MS require pharmacologic intervention for the condition during the course of their disease progression.3 Furthermore, the suicide rate among depressed MS patients is significantly higher than that of the general population and that of patients with MS not suffering from depression.2 4
The high incidence of depression in MS may reflect several elements of both MS disease and its treatment. The manifestation of depression may be related to the natural history of MS, resulting from neuronal damage and/or immune dysfunction, which may induce or propagate depressive symptoms.1 5–7 Depression can also be a psychological reaction to the lifestyle changes that occur when patients face the reality of living with a chronic disease such as MS.1 5–7 Finally, depression has been considered as an adverse event, caused by disease-modifying therapies (DMTs) for MS8–10; many prescribers consider the likelihood of a DMT to cause depression when making treatment decisions.
The most commonly used DMTs for the treatment of MS are interferon beta (IFNβ)−1a (Rebif, EMD Serono, Inc, Rockland, MA; and Avonex, Biogen Idec, Cambridge, MA), IFNβ-1b (Betaseron, Bayer Health-Care Pharmaceuticals, Montville, NJ; and Extavia, Novartis Pharmaceuticals, East Hanover, NJ), and glatiramer acetate (GA) (Copaxone, Teva Pharmaceutical Industries Ltd, North Wales, PA). A common perception among prescribers of DMTs for MS is that IFNβ treatment is more likely than GA to increase depression during the course of MS treatment.11–13 Physicians were alerted that there seemed to be an association between depression and interferon compounds from the pivotal trials leading to the approval of IFNβ for the treatment of relapsing-remitting MS (RRMS).8–10 In addition, neurologists often associate interferon therapy with depression based on information reported in the internal medicine arena regarding high-dose interferon alpha (IFNα) therapy in the management of hepatitis C14 and in cancer chemotherapy.15 Therefore, in order to avoid exacerbating depressive symptoms, newly diagnosed patients with MS who have a history of depression are often placed on GA therapy from the onset of treatment.16 In light of this perception, the purpose of this study was to determine whether there is a relationship between DMT type and depression.
Methods
Patients
The Baptist Hospital East MS Center in Louisville, Kentucky, was a comprehensive clinic that provided diagnosis and treatment services to, and conducted research studies of, the MS population in Louisville and the surrounding counties in Kentucky and southern Indiana from 1996 to 2009. The medical records of patients with RRMS who were examined at the MS Center from 2000 to 2007 were reviewed to identify patients who, after initiating a DMT, had remained on the same DMT for up to 4 years. Only newly diagnosed patients with RRMS who started on IFNβ or GA were considered candidates for this retrospective analysis.
From a review of the records of approximately 500 patients, we identified 112 patients with RRMS who had remained on a single therapy (either an IFNβ or GA) from the initiation of therapy throughout the course of treatment for up to 4 years. Most of the other patients followed at the MS Center had presented to the Center after initiating therapy or were not diagnosed with RRMS at the time of presentation and were not considered candidates for the study. Patients who had stopped or changed therapy were not candidates for the study. Most of the changes in therapy were related to breakthrough clinical disease or injection intolerance. Notably, there were no cases of suicide or severe depression in the patient population from 2000 to 2007.
The medical records of patients with RRMS who were examined at the MS Center from 2000 to 2007 and whose records were maintained at University Neurologists, PSC, were included in the analysis. In 2000, routine screening for depression was initiated at the MS Center, and all MS patients were assessed using the Beck Depression Inventory (BDI) at all follow-up visits. Of the patients treated with a single therapy (either an IFNβ or GA) for up to 4 years, only those patients who were administered the BDI within 6 weeks of starting their DMT were included in this study (n = 67). The University of Louisville Investigational Review Board reviewed the study and approved its conduct using de-identified data from the records review.
Study Design
The patients seen at the MS Center each received a comprehensive analysis. This analysis included physical assessments (Timed 25-Foot Walk, Box and Blocks, Nine-Hole Peg Test, Expanded Disability Status Scale [EDSS]) and cognitive and psychological assessments (BDI, Modified Fatigue Impact Scale [MFIS], Repeatable Battery for the Assessment of Neuropsychological Status [RBANS], 36-item Short Form Health Status Survey [SF-36]). Patients were routinely reevaluated at 6-month intervals, when they repeated the assessments except for the RBANS, which was repeated at 2-year intervals or when needed.
Each patient had a BDI score recorded at treatment initiation and at 6, 12, 24, 36, and 48 months following treatment initiation. In order to exclude variation from the initial patient visit to 48 months in the analysis of BDI score, only those patients who had completed a BDI within 6 weeks of starting their DMT were included in the analysis. Baseline values were set as the initial BDI score within the first 6 weeks after treatment initiation.
Assessments
Beck Depression Inventory (BDI-1A)
Version 1A of the BDI was used in this study. The total BDI-1A score, a sum of the scores for 21 questions based on a 4-point Likert scale (possible score of 0–3 for each question) assessing the severity of patients' depression during the previous week, was obtained at each patient visit. For each individual item, a score of 0 denotes “I am not depressed” while a score of 3 denotes “I am extremely depressed.” As a general framework, a total score between 5 and 9 reflects normal individuals who are generally depression-free; a score between 10 and 18 is considered moderate depression; a score between 19 and 29 denotes moderate-to-severe depression; and a score between 30 and 63 indicates severe depression.
Other Measurements
Measurements were also made for the Timed 25-Foot Walk, Nine-Hole Peg Test, Box and Blocks, MFIS, Tinetti Balance Assessment Tool, and EDSS.
Statistical Analysis
Patients were measured at baseline and at months 6, 12, 24, 36, and 48 from the baseline measurement. Because of the violations of normality, raw baseline scores and change scores for subsequent measurements are presented with medians and interquartile ranges, and group differences were evaluated using the Exact Wilcoxon test. Statistical analyses were performed using R Version 2.13.1.17
Results
Patient Baseline Characteristics
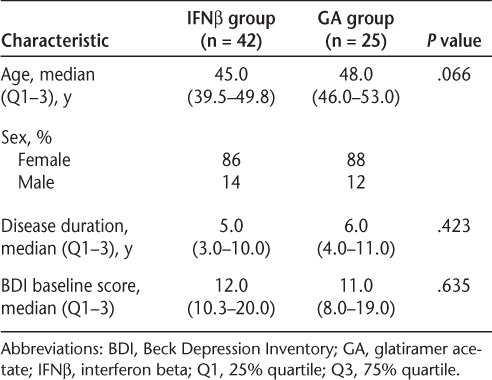
A total of 112 patients were diagnosed with RRMS and remained exclusively on either IFNβ (n = 80) or GA (n = 32) during the study period. All patients completed an initial BDI and were assessed using the BDI at all follow-up visits. Of the 112 patients, a total of 67 patients had completed a BDI within 6 weeks of initiating the DMT (IFNβ: n = 42; GA: n = 25) (Figure 1); only these patients were included in the analysis. Baseline characteristics were similar between the two treatment groups with respect to gender, age, and total BDI score (Table 1). The IFNβ group was 86% female, with a median age of 45 years (range, 39.5–49.8 years) and a median disease duration of 5 years (range, 3–10 years). Patients in the IFNβ group were moderately depressed at the start of the study, with a median total BDI score of 12 (range, 10.3–20.0). The GA group was 88% female, with a median age of 48 years (range, 46–53 years) and a median disease duration of 6 years (range, 4–11 years). The patients in this group were also moderately depressed, with a median total BDI score of 11 (range, 8–19).
Patients included in the analysis
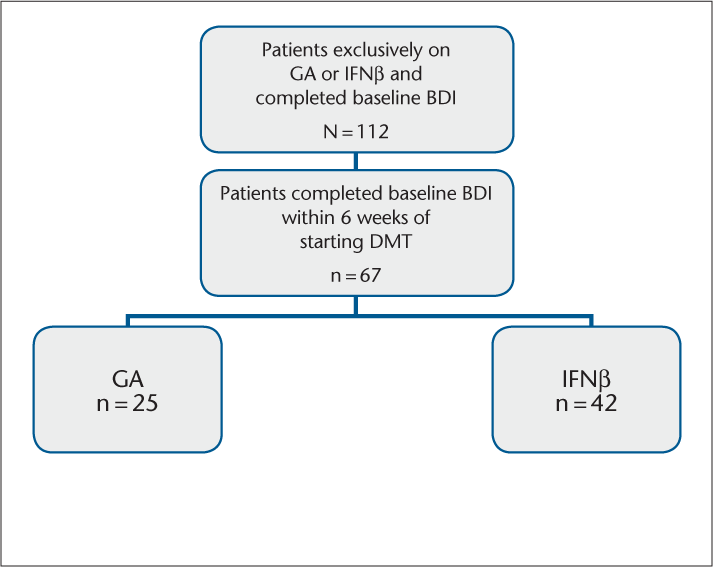
Baseline characteristics of patients completing the BDI within 6 weeks of initiating therapy (n = 67)
Median and Mean Changes in BDI Scores from Baseline
Scores on the BDI improved from baseline in the IFNβ treatment group, with a median improvement of 2 points at 6 and 12 months and a median improvement of 2.5 points at 48 months (Table 2). Scores on the BDI also improved from baseline in the GA treatment group, with a median improvement of 1 point at 6 and 12 months and a median improvement of 4 points at 48 months. There were no statistically significant differences in the median change in the BDI score from baseline to 48 months between the two treatment groups, except for the median change from baseline at 36 months (IFNβ group, +2-point change; GA group, −4-point change; P = .030) (Table 2). This difference was no longer present at the 48-month assessment.
Median and mean change from baseline in BDI scores in the IFNβ and GA treatment groups (n = 67)
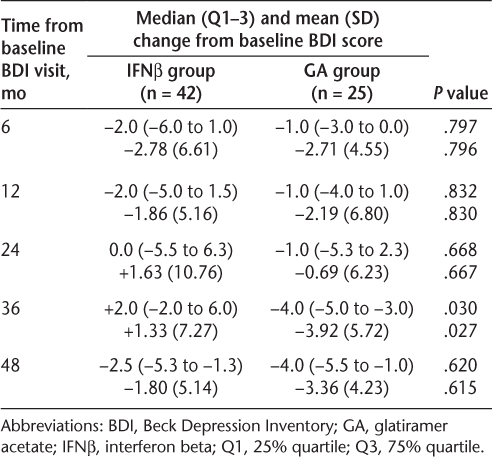
Furthermore, no statistically significant differences in mean change in BDI score from baseline were observed between groups for any other time interval, with the sole exception of the 36-month interval, at which point the mean changes in BDI score in the IFNβ and GA groups were +1.33 ± 7.27 and −3.92 ± 5.72, respectively (P = .027) (Table 2). This difference was no longer present at the 48-month assessment.
Use of Concomitant Antidepressant Medication
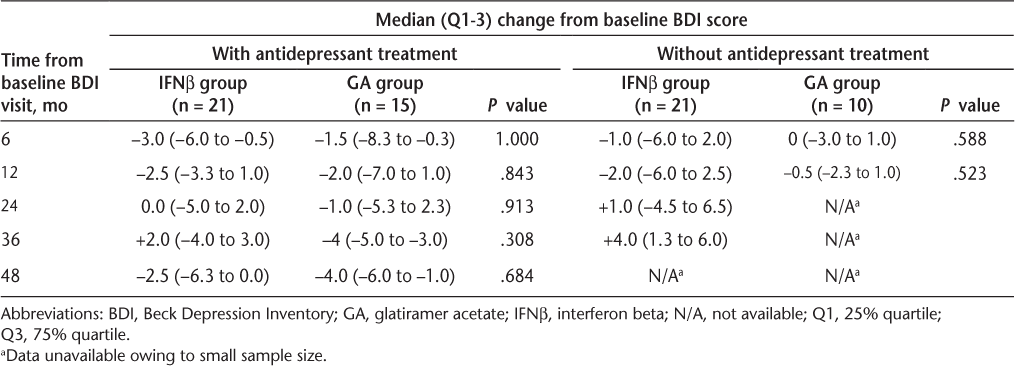
In order to determine whether concomitant antidepressant medication could have influenced these results, a subgroup analysis was performed to determine whether there were any differences in the change in BDI score between those patients who were treated with antidepressant medication and those who were not. At all time intervals examined, there were no significant differences in median change in BDI score from baseline between patients within the IFNβ group who were receiving anti-depressants and patients in the IFNβ group who were not. The changes from baseline between the two IFNβ subgroups fluctuated across time points; however, the differences between the two IFNβ subgroups did not significantly differ at any time point (data not shown). Similarly, no significant differences in median change in BDI score from baseline were observed between antidepressant-treated and antidepressant-free patients within the GA group (data not shown), although, as in the IFNβ subgroups, the changes from baseline between the GA subgroups did fluctuate over time.
Lastly, no significant differences in median change in BDI score from baseline were observed at any time points between patients receiving antidepressants in the IFNβ subgroup and those receiving antidepressants in the GA subgroup (Table 3). Similar results were observed when comparing patients who were antidepressant-free in the IFNβ subgroup with those who were antidepressant-free in the GA subgroup. Fifty percent of patients in the IFNβ subgroup received antidepressants during the study period, compared with 60% of those in the GA subgroup.
Median change from baseline in BDI scores in patients with and without antidepressant treatment
Discussion
This study was performed in order to assess any differences between IFNβ and GA in their propensity to cause depression. Patients with and without depression at baseline demonstrated no significant differences in the change in BDI score over 48 months by DMT type. Interestingly, these results are in sharp contrast to those of several previous studies that included published case reports and suggested that IFNβ treatment is associated with an increased incidence of depression in MS patients,1 11–13 but are in agreement with those of several studies that did not find any relationship between DMT type and depression.18–20
The results of this study also revealed a small, non-significant decrease in median BDI scores in both treatment groups over 48 months, suggesting a slight improvement in depressive symptoms while on DMT treatment, which was not dependent on concomitant antidepressant treatment. For example, there were no significant differences within both the IFNβ group and the GA group in BDI scores between those patients receiving antidepressants and those who were not. This is in accordance with an analysis of information from a Canadian database, which also found no difference in antidepressant use in MS patients treated with IFNβ-1a versus GA.3
It is important to consider the results of this study in the context of its limitations, namely, small study size and a retrospective design that establishes the potential for bias. Because of physicians' perceptions that depression is associated more closely with IFNβ than with GA treatment, patients with a history of depression may have been disproportionately prescribed GA treatment. This could have led to biased estimates of depression, as patients more vulnerable to depression may have been selectively treated with GA. As a result, if some of the patients in the GA group already had a history of depression, then the impact of GA on depression may not have been readily noticeable. Conversely, if there were a disproportionate number of patients without a history of depression in the IFNβ group, then that group may have been less susceptible to IFNβ-induced depression and might appear more stable over time. However, since the baseline BDI scores were comparable in both treatment groups, the possibility of introducing this bias was minimal. Additionally, patients included in this study were required to remain on therapy. Only patients with RRMS who had remained on a single therapy (either an IFNβ or GA) from the initiation of therapy throughout the course of treatment for up to 4 years were included in the study. Importantly, there was no evidence that a switch of therapy was related to depressive symptoms; only injection issues or poor response was associated with a change in therapy. It is conceivable that patients discontinued therapy in a way that is related to depression or that, for some other reason, the studied population is not representative of all patients receiving DMTs for MS. Any future studies on depression and MS treatment should involve larger sample sizes and a randomized treatment allocation to minimize the introduction of bias. Finally, another limitation of this study was that differences in disability between the IFNβ group and the GA group were unknown; more severely disabled patients may not improve in depression as well as less disabled patients.
Nevertheless, these are interesting data that warrant this evaluation on a larger scale. The recent BEYOND (Betaseron® Efficacy Yielding Outcomes of a New Dose) trial, which prospectively compared the efficacy and safety of two different doses of IFNβ-1b with GA, found no significant differences in the incidence of depression or depression-related adverse events among patients with MS (N = 2447) randomized to IFNβ-1b 500 μg, IFNβ-1b 250 μg, or GA.21 22 In addition, long-term findings from the PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) trial, which prospectively compared the efficacy and safety of two different doses of IFNβ–1a with placebo, found no significant differences in the incidence of depression or depression-related adverse events among patients with MS (N = 560) randomized to IFNβ-1a 22 μg, IFNβ-1a 44 μg, or placebo over time.23 While BEYOND and PRISMS were not specifically designed to examine depression as an outcome, these results are supportive of the outcomes observed in the current study.
The results of this study are important in light of the common perception that IFNβ is associated with an increase in depressive symptoms.11–13 Depression is a common problem in MS. The difficulty is in determining whether the incidence or degree of depression is affected by IFNβ therapy. The published literature has a void of information on this aspect because most studies have not included depression scores in their analyses. It has been well documented that depression in patients with MS is related to a poorer quality of life, diminished positive outlook, and, most importantly, reduced adherence to treatment.24–27 Therefore, a history of depression has led to many prescribers avoiding IFNβ therapy for their patients, based on findings in the pivotal interferon studies that suggested a possible increase in depressive symptoms after starting IFNβ therapy.1,11 This study as well as other comparison studies have not supported those earlier findings.18–23 There is mounting clinical evidence that IFNβ therapy is not likely to induce depression in MS patients.1 Treating depression pharmacologically or with cognitive-behavioral therapy is usually very effective in MS patients.28–30 Based on the findings of this study, initial DMT selection could be broadened with close monitoring of depressive symptoms.
PracticePoints
Many prescribers consider the likelihood of a disease-modifying therapy (DMT) for MS to cause depression when making treatment decisions.
Because of physicians' perceptions that depression is associated more closely with interferon beta (IFNβ) than with glatiramer acetate (GA), MS patients with a history of depression may have been disproportionately prescribed GA therapy.
Our findings show that neither depression status nor development of depression was associated with DMT type; neither IFNβ nor GA therapy appears to exacerbate depressive symptoms in patients with relapsing-remitting MS.
Acknowledgments
We would like to thank Precept Medical Communications of Warren, New Jersey, for editorial assistance.
References
Siegert RJ, Abernethy DA. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry. 2005; 76: 469–475.
Caine ED, Schwid SR. Multiple sclerosis, depression, and the risk of suicide. Neurology. 2002; 59: 662–663.
Patten SB, Williams JV, Metz LM. Anti-depressant use in association with interferon and glatiramer acetate treatment in multiple sclerosis. Mult Scler. 2008; 14: 406–411.
Jose SM. Psychological aspects of multiple sclerosis. Clin Neurol Neurosurg. 2008; 110: 868–877.
Wallin MT, Wilken JA, Turner AP, Williams RM, Kane R. Depression and multiple sclerosis: review of a lethal combination. J Rehabil Res Dev. 2006; 43: 45–62.
Patten SB, Metz LM. Depression in multiple sclerosis. Psychother Psychosom. 1997; 66: 286–292.
Ehde DM, Bombardier CH. Depression in persons with multiple sclerosis. Phys Med Rehabil Clin N Am. 2005;16:437–448, ix.
Avonex [prescribing information]. Cambridge, MA: Biogen Idec Inc; 2012.
Rebif [prescribing information]. New York, NY: Pfizer Inc; 2011.
Betaseron [prescribing information]. Montville, NJ: Bayer HealthCare Pharmaceuticals Inc; 2010.
Arnett PA, Randolph JJ. Longitudinal course of depression symptoms in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2006; 77: 606–610.
Goeb JL, Cailleau A, Laine P, et al. Acute delirium, delusion, and depression during IFN-beta-1a therapy for multiple sclerosis: a case report. Clin Neuropharmacol. 2003; 26: 5–7.
Pandya R, Patten S. Depression in multiple sclerosis associated with interferon beta-1a (Rebif). Can J Psychiatry. 2002; 47:686.
Udina M, Castellví P, Moreno-Espana J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012; 73: 1128–1138.
Dutcher JP, Logan T, Gordon M, et al. Phase II trial of interleukin 2, interferon alpha, and 5-fluorouracil in metastatic renal cell cancer: a Cytokine Working Group study. Clin Cancer Res. 2000; 6: 3442–3450.
Wilken JA, Sullivan C. Recognizing and treating common psychiatric disorders in multiple sclerosis. Neurologist. 2007; 13: 343–354.
R Development Core Team. R: a language and environment for statistical computing. 2012.
Patten SB, Fridhandler S, Beck CA, Metz LM. Depressive symptoms in a treated multiple sclerosis cohort. Mult Scler. 2003; 9: 616–620.
Patten SB, Metz LM. Interferon beta1a and depression in secondary progressive MS: data from the SPECTRIMS Trial. Neurology. 2002; 59: 744–746.
Feinstein A. Multiple sclerosis, disease modifying treatments and depression: a critical methodological review. Mult Scler. 2000; 6: 343–348.
O'Connor P, Filippi M, Arnason B, et al. 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol. 2009; 8: 889–897.
Schippling S. Impact of depression in multiple sclerosis during treatment with interferon beta-1b and glatiramer acetate in the multinational BEYOND study [abstract]. Mult Scler. 2009; 15(suppl):S118.
PRISMS Study Group and the University of British Columbia MS/MRI Analyses Group. PRISMS-4: Long-term efficacy of interferon-beta-1a in relapsing MS. Neurology. 2001; 56: 1628–1636.
DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000; 160: 2101–2107.
Lynch SG, Kroencke DC, Denney DR. The relationship between disability and depression in multiple sclerosis: the role of uncertainty, coping, and hope. Mult Scler. 2001; 7: 411–416.
Hart S, Fonareva I, Merluzzi N, Mohr DC. Treatment for depression and its relationship to improvement in quality of life and psychological well-being in multiple sclerosis patients. Qual Life Res. 2005; 14: 695–703.
Lester K, Stepleman L, Hughes M. The association of illness severity, self-reported cognitive impairment, and perceived illness management with depression and anxiety in a multiple sclerosis clinic population. J Behav Med. 2007; 30: 177–186.
Mohr DC, Hart SL, Fonareva I, Tasch ES. Treatment of depression for patients with multiple sclerosis in neurology clinics. Mult Scler. 2006; 12: 204–208.
Sollom AC, Kneebone II. Treatment of depression in people who have multiple sclerosis. Mult Scler. 2007; 13: 632–635.
Walker ID, Gonzalez EW. Review of intervention studies on depression in persons with multiple sclerosis. Issues Ment Health Nurs. 2007; 28: 511–531.
Financial Disclosures: Dr. Jones is a former employee of Bayer HealthCare Pharmaceuticals, Inc. The other authors have no conflicts of interest to disclose.
Funding/Support: The editorial assistance provided by Precept Medical Communications was funded by Bayer HealthCare Pharmaceuticals, Inc.







