Publication
Research Article
International Journal of MS Care
Progressive Multifocal Leukoencephalopathy Risk Perception in Patients Considering Natalizumab for Multiple Sclerosis
Author(s):
Abstract
Background:
Progressive multifocal leukoencephalopathy (PML) remains a concern when considering natalizumab for multiple sclerosis (MS) treatment. Extensive research has identified factors that increase PML risk, and it is important that providers and patients accurately understand risk to make appropriate benefit-risk decisions.
Methods:
One hundred adult US patient-candidates for natalizumab therapy were questioned about their PML risk perception, the maximum PML risk they deemed acceptable, and sources of information used to understand risk. Differences in group distributions were compared.
Results:
Patients estimated their potential PML risk from 0.1% to 87% (mean, 31.5%). Maximum PML risk deemed acceptable ranged from 0.1% to 45% (mean, 14.5%). Actual risk (mean, 0.01%), based on published risk estimates, was calculated as a function of time receiving therapy, anti–John Cunningham virus antibody index, and previous use of immunosuppressants. The sexes perceived their risks similarly and had similar risk acceptance. Patient perception of PML risk increased with age, whereas willingness to accept risk remained similar among all ages. Higher levels of education correlated with more accurate risk perception and lower risk tolerance. Neither risk perception nor tolerance was correlated with disability level. Sixty-three percent of patients indicated that their primary/referring physician’s concern level regarding potential risk of PML during the benefit-risk discussion was their main source of information about risk.
Conclusions:
Patients with MS substantially overestimated their PML risk, often by three orders of magnitude. Patients with MS could benefit from accurate risk education, and providers could play an essential role in presenting PML risk information in a manner understandable to each individual patient.
Multiple sclerosis (MS) is an autoimmune disorder that has the potential to cause major disability. Medications used to treat MS are often referred to as disease-modifying therapies (DMTs) because they have demonstrated the potential to substantially alter the course of MS. In fact, one of the more recent measures of MS therapeutic efficacy can be expressed as no evidence of disease activity–3 (NEDA-3), where successful treatment prevents measurable relapse, magnetic resonance imaging activity, and accrual of MS-related disability. Although this is a common treatment goal from an efficacy perspective, both the patient with MS and the treating provider must also weigh the risk of the DMT.
All medications are taken for their potential benefit, but they also have the potential to do harm. Without an accurate understanding of the risks and benefits of each medication, it is difficult (if not impossible) for patients and health care providers to make an accurate therapeutic decision. There is now a plethora of DMTs for the treatment of MS, and making an appropriate DMT choice is becoming more challenging for patients and clinicians, even more so when serious adverse events are associated with some DMTs. A notable component of DMT choice is each individual’s perception of risk.
It has been suggested that the perception of risk is based more on fear than statistics,1 and, unfortunately, evidence suggests that knowledge of the actual risks may have little impact on public perception and response to risk.2 In a chronic disease such as MS, the clinician’s role is first to fully understand the actual risk, and then to educate patients and direct the interaction to shared decision making, a cooperative approach whereby patients and clinicians mutually agree on each treatment decision. Shared decision-making is particularly relevant when there are several reasonable treatment options, each with a different safety and efficacy profile.3 , 4 In chronic conditions, where lifelong treatment may be needed, studies have demonstrated that shared decision making can enhance patients’ satisfaction, treatment compliance, and overall well-being.5
Natalizumab is a highly efficacious medication6 , 7 for MS and has been associated with NEDA in many patients.8 It was approved by the US Food and Drug Administration in 2004, but shortly thereafter, progressive multifocal leukoencephalopathy (PML) was discovered as a major potential adverse event associated with natalizumab use.9 Since then, substantial efforts have been devoted to identifying factors affecting the risk of developing natalizumab-associated PML and estimating the risk to help physicians make individualized benefit-risk assessments.10 This article reports the PML risk perceptions of patients as they consider natalizumab to treat their MS.
Methods
As part of routine clinical care in a large single-provider MS practice, during a consultation focused on the decision-making process related to potential initiation of treatment with natalizumab, a discussion of PML risks with adult patients with MS was recorded in the patient’s medical record. Patients were questioned about their perception of their PML risk, the maximum PML risk they deemed acceptable for complete control of their MS, and the sources of information they used to understand risk (patients were allowed to list more than one source of information). Specifically, the questions were as follows: “What do you think your personal risk of PML may be?”; “What level of risk would you see as acceptable for yourself?”; and “What source did you use to get the information on this?” All responses were recorded in the patients’ medical records. The answers to the first 2 questions were analyzed and reported as the patients’ perceived risk and risk tolerance. Actual PML risk was calculated using the algorithm published by Ho et al10 and was based on available anti–John Cunningham virus (JCV) antibody index values, months of exposure to natalizumab, and presence or absence of immunosuppressant use history.
Patients’ reactions to accurate PML risk information were recorded. A retrospective medical record review of the risk perception responses from 100 randomly selected US patient-candidates for natalizumab treatment was performed.
Differences in group distributions were compared using the t test or analysis of variance. This retrospective medical record review was approved by the institutional review board of the University of Southern California.
Results
One hundred adult patients with MS—potential candidates for natalizumab treatment—who were questioned about their perception of and tolerance for PML risk were included in this study. Most patients (n = 81) were naïve to natalizumab. The remaining 19 patients were new patients to the clinic who were considering whether to continue natalizumab treatment. Patients started taking natalizumab between 2008 and 2018 and were questioned between 2013 and 2018. Patient demographics are presented in Table S1, which is published in the online version of this article at ijmsc.org.
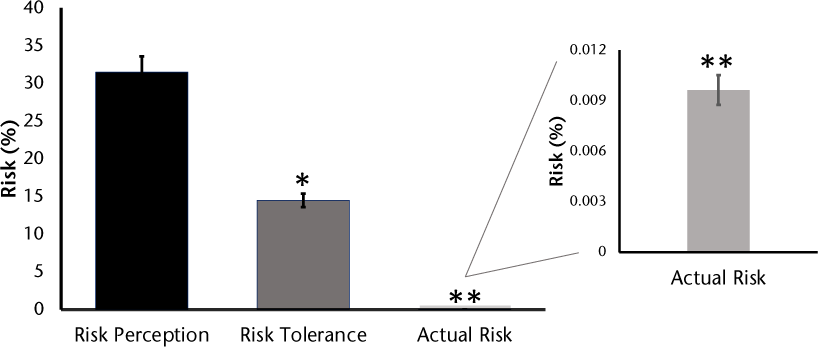
Figure 1 illustrates the patients’ estimated perception of PML risk if they decided to receive/continue natalizumab (0.1%–87%; mean, 31.5%), the maximum PML risk they deemed acceptable (0.1%–45%; mean, 14.5%), and the actual PML risk (0.001%–0.03%; mean, 0.01%). Actual risk was calculated based on the risk factors published by Ho et al.10 For example, a patient taking natalizumab for 9 months with an anti-JCV antibody index of 1.3 and no previous immunosuppressant use would have a risk of 0.1/1000 (equal to 0.01%).
Progressive multifocal leukoencephalopathy risk perception and tolerance versus actual risk
Perceived PML risk increased with age, but risk acceptance was similar among all ages (Figure S1). Table 1 presents risk based on sex, ethnicity, educational level, and MS disease duration (time since diagnosis). Men and women perceived their PML risk similarly (28% and 34%, respectively) and were willing to accept a similar level of risk (13% and 16%, respectively). Actual PML risk was 0.009% for women and 0.011% for men. Educational level had a significant effect on risk estimates and tolerance. Patients were categorized based on their highest level of completed or partially completed education. Those with higher levels of education (high school degree vs bachelor’s degree vs master’s degree) estimated more accurate (lower) risk (44%, 33%, and 7%, respectively). The same significant trend was observed for risk tolerance (19%, 15%, and 8%, respectively). Actual PML risk was 0.012% for high school degree, 0.010% for bachelor’s degree, and 0.006% for master’s degree. Black, White, and Hispanic patients estimated PML risk and risk tolerance at similar levels (34%, 33%, and 35%, respectively), whereas Asian American patients estimated a significantly lower risk value of 3%. There were only 6 Asian American patients in this cohort, and they had a higher level of education than the other 3 groups. Actual risk values were 0.011%, 0.009%, 0.015%, and 0.006% for Black, White, Hispanic, and Asian American patients, respectively. Neither risk perception nor tolerance was correlated with disability level (as measured by Expanded Disability Status Scale scores).
Patient Risk Stratified by Sex, Ethnicity, Educational Level, and MS Disease Duration
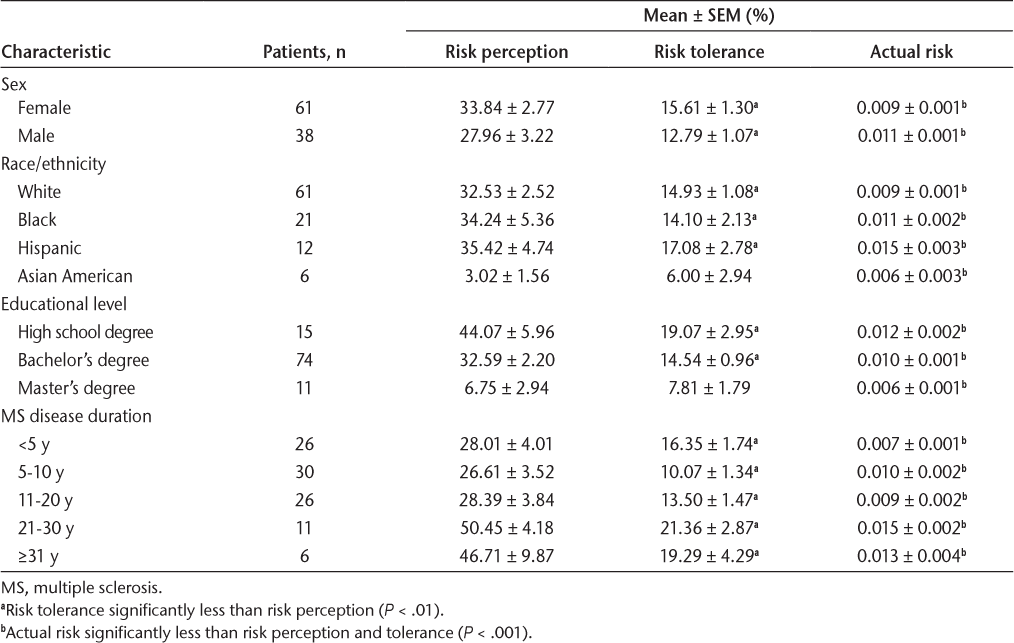
Table S2 reports the primary source(s) that patients used to form their perception. Most patients (63%) indicated that their primary/referring physician’s concern level regarding potential risk of PML during the benefit-risk discussion was their main source of information about risk. This group of patients also had the greatest risk perception (42%) despite having low actual risk (0.014%). Although only 11 patients reported using the scientific literature as their source of risk information, this group had the most accurate assessment of their risk (0.43% perceived and 0.003% actual). Interestingly, this is the only group that was willing to accept a higher risk than their perceived risk. Other common sources of information included discussions with fellow patients with MS, Biogen patient program or phone call, and the internet. In addition, 2 patients thought that their anti-JCV antibody index score was a reflection of their PML risk.
Discussion
These data demonstrate that patients with MS significantly overestimate their risk of PML associated with natalizumab treatment. Patients, on average, thought that they had a 31.5% chance of acquiring PML while taking natalizumab when their actual risk was 0.01%. In addition, patients reported a willingness to accept an average risk of 14.5%, which is still greater than 3 orders of magnitude higher than their actual risk of 0.01%. The possible reasons behind a greater than 1000-fold difference between actual risk and reported perceived or acceptable risk values are unclear.
One potential key aspect of the data presented herein is the context in which patients were asked about risk. Specifically, patients were asked about the level of risk they would accept for nearly complete disease control associated with a well-known medication of high efficacy (Tysabri).11 A different approach was recently published by Fox et al12 where patients were asked about PML risk preference and acceptance for a hypothetical therapy with a fixed benefit of 50% reduction in clinical relapse rate and a 30% reduction in disability progression (the approximate efficacy of many of the currently used MS therapies). That survey used an iterative algorithm, starting with a risk of 1:1000, to determine the maximum risk tolerance for each survey respondent. Median risk tolerance for PML was 1:1,000,000. Although these data stand in stark contrast to the data presented herein, an expectation of partial efficacy versus nearly complete efficacy likely leads to a significant difference in risk tolerance.
Moreover, it has been our experience that a fully informed, educated decision on DMT choice is nearly impossible until the DMT in question has been actually started by the patient with MS. Only then can the patient accurately understand the benefit (or lack thereof) of the medication. This has been supported by hundreds of testimonials from patients with MS (documented in patients’ medical records) on how different their views on the DMT were before and after the medication had been started. Interestingly, Heesen et al13 , 14 reported risk tolerance thresholds whereby natalizumab-treated patients would discontinue natalizumab for the risk of PML. The initial set of data13 reported that 29% of patients would not discontinue natalizumab use until the risk was 10% or greater. A subsequent study14 reported that 40% of natalizumab-treated patients would not discontinue natalizumab use at a PML risk of 25%.
After patients stated their risk perception and tolerance, they were asked about the source(s) they used to help make their decisions. The lower range of the risk perception reports—and as it happens the most accurate ones—were associated with a higher level of education and the use of reliable sources of information (scientific literature). It is remarkable that those patients, who more accurately estimated their risks, were willing (consciously) to take a similar or higher risk compared with their actual risk.
Patients who were the least accurate in their estimates cited that they judged by the referring physician’s concern level regarding potential risk of PML. Heesen et al13 , 14 reported that neurologists are more risk averse than their patients. Thus, it may not be surprising that patients who primarily base their decision on their interpretation of their local neurologist’s concern might overestimate the risk. High risk estimates were also observed after interaction with Biogen via patient programs or phone calls, which may reflect the company’s efforts to remain fair-balanced and ensure that PML risk is known to support benefit-risk discussions. Finally, 2 patients with the highest risk perceptions of 70% and 87% were judging by their anti-JCV antibody index numbers, which were 0.70 and 0.87, respectively. This is an unfortunate lack of understanding of the anti-JCV antibody component of PML risk stratification.
Understanding of risk is multifactorial and includes factors such as emotions, personal values, social pressures, environmental barriers, and economic constraints.15 Therefore, it may not be surprising that both an underestimation and an overestimation of risk have been reported in the literature.16 , 17 However, understanding risk is fundamental to evidence-based decision making18 and, therefore, should be considered an important aspect of any therapy with the potential for significant adverse events. In our experience, having the patient verbalize their perceived and personally acceptable risk, followed by a discussion of their actual risk, has been effective in helping patients better understand their true risk. This could be taken a step further by following the suggestion of Fox and Rudick19 to compare natalizumab-associated PML risk with more familiar catastrophic risks, such as the lifetime risk of death from an airplane crash (1:20,000), drowning (1:8942), fire or smoke exposure (1:1116), or an automobile accident (1:100). This appropriate framing of risk could be valuable for the patient as well as the provider. However, more research needs to be conducted on effective risk communication in MS.
A limitation of this report is that data gathered from a single center may not be representative of the general US or global population. It is possible that regional and cultural differences exist in risk understanding and risk tolerance. However, given the magnitude of the risk discrepancy reported herein, we hypothesize that increased risk understanding would likely be broadly relevant. As with any retrospective study, data quality can be a concern. Specifically, we did not analyze data based on previous DMT because the data quality from transfer patients was not deemed by us to be of high enough reliability. However, because this was a single site, the data reported herein are consistent and reliable for the outcomes reported.
In conclusion, patients with MS in this study significantly overestimated their PML risk, often by 3 orders of magnitude. Most patients in this cohort were willing to accept a PML risk much higher than their actual risk. This discrepancy between real and perceived risk indicates an important need for education. Patients could benefit from accurate risk education, and providers could play an essential role in presenting PML risk information in a manner understandable to each individual patient.
PRACTICE POINTS
Natalizumab is an effective medication for MS but is associated with progressive multifocal leukoencephalopathy (PML). It is important that patients and providers understand the risk of PML so that they can make educated risk-benefit decisions.
This study demonstrates that patients with MS commonly overestimate their risk of PML on natalizumab therapy, often by 3 orders of magnitude.
This discrepancy between real and perceived risk highlights an important need for education.
References
Inman WHW. Risks in medical intervention. In: Cooper MG, ed. Risk: Man-made Hazards to Man . Clarendon Press; 1985: 35– 53.
Alaszewski A, Horlick-Jones T. How can doctors communicate information about risk more effectively? BMJ . 2003; 327: 728– 731.
Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997; 44: 681– 692.
Charles C, Whelan T, Gafni A. What do we mean by partnership in making decisions about treatment? BMJ . 1999; 319: 780– 782.
Joosten E, DeFuentes-Merillas L, de Weert G, . Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008; 77: 219– 226.
Miller DH, Khan OA, Sheremata WA, . A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003; 348: 15– 23.
Polman CH, O’Connor PW, Havrdova E, . A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006; 354: 899– 910.
Perumal J, Fox RJ, Balabanov R, . Outcomes of natalizumab treatment within 3 years of relapsing-remitting multiple sclerosis diagnosis: a prespecified 2-year interim analysis of STRIVE. BMC Neurol. 2019; 19: 116.
Stuve O, Bennett JL. Pharmacological properties, toxicology and scientific rationale for the use of natalizumab (Tysabri) in inflammatory diseases. CNS Drug Rev. 2007; 13: 79– 95.
Ho PR, Koendgen H, Campbell N, . Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol. 2017; 16: 925– 933.
Tysabri® (natalizumab) [prescribing information]. Biogen Inc; 2019.
Fox RJ, Cosenza C, Cripps L, . A survey of risk tolerance to multiple sclerosis therapies. Neurology. 2019; 92: e1634– e1642.
Heesen C, Kleiter I, Nguyen F, . Risk perception in natalizumab-treated multiple sclerosis patients and their neurologists. Mult Scler. 2010; 16: 1507– 1512.
Heesen C, Kleiter I, Meuth SG, . Benefit-risk perception of natalizumab therapy in neurologists and a large cohort or multiple sclerosis patients. J Neurol Sci. 2017; 376: 181– 190.
Weinstein ND. What does it mean to understand risk? evaluating risk comprehension. Monogr Natl Cancer Inst. 1999; 25: 15– 20.
Everett B, Salamonson Y, Rolley JX, Davidson PM. Underestimation of risk perception in patients at risk of heart disease. Eur J Cardiovasc Nurs. 2016; 15: e2– e9.
Stier MW, Lodhia N, Jacobs J, . Perceptions of risk and therapy among patients with Barrett’s esophagus: a patient survey study. Dis Esophagus. 2017; 31: 1– 7.
Bell NR, Dickinson JA, Grad R, Singh H, Kasperavicius D, Thombs BD. Understanding and communicating risk: measures of outcome and the magnitude of benefits and harms. Can Fam Physician. 2018; 64: 181– 185.
Fox RJ, Rudick RA. Risk stratification and patient counseling for natalizumab in multiple sclerosis. Neurology. 2012; 78: 436– 437.
Financial Disclosures: Dr Berkovich has received speaker fees and/or advisory board fees from Alexion, Bayer, Biogen, Celgene, Genentech, Novartis, and Sanofi. Dr Riddle is an employee of Biogen. The other authors declare no conflicts of interest.
Funding/Support: None.
Disclaimer: Biogen reviewed and provided feedback on the manuscript to the authors. The authors had full editorial control of the manuscript and provided their final approval of all content.
Prior Presentation: Aspects of this study have been presented in poster form at the American Academy of Neurology Annual Meeting; April 21-27, 2018; Los Angeles, California.







