Practice Points
- Sleep disturbance is highly prevalent in people with multiple sclerosis (MS) and is often associated with dysfunctional sleep beliefs.
- When people with MS report poor sleep, clinicians should consider referring to sleep medicine to evaluate and treat underlying causes, with consideration for MS symptoms that exacerbate sleep disturbance.
- Assessments and interventions to improve sleep quality and manage dysfunctional sleep beliefs in people with MS may improve health-related quality of life and functioning. Cognitive behavioral therapy for insomnia targets both factors and has been demonstrated to be a modifiable and efficacious treatment in this population.
Research findings suggest that approximately 50% to 70% of people with multiple sclerosis (MS) experience sleep disturbance.1-4 This prevalence makes them more likely to experience sleep problems than other populations with chronic illness and up to 4 times more likely than the general population.1,2 In people with MS, poor sleep quality is well established as a detriment to health-related quality of life and functioning (HRQOL/F), which is a broad, multidimensional construct that considers how an individual perceives the influence of their physical, mental, and social health and health status on their ability to live a fulfilling life.5 In people with MS, poor sleep quality has been associated with decreased HRQOL/F via cognitive dysfunction, fatigue, and depression.1,2,6,7 Most studies assessing sleep quality and HRQOL/F in people with MS have relied on self-report sleep measures, though some have utilized polysomnography.8-12 People with MS and comorbid sleep disorders such as insomnia or sleep apnea have poorer HRQOL/F than people with MS and no comorbid sleep disorders.13 Results from studies using the Pittsburgh Sleep Quality Index (PSQI) found significant associations between poorer sleep quality and worse HRQOL/F and disability-related outcomes.8,9,14-21 Vitkova and colleagues10 report an indirect relationship between disability and PSQI scores, such that poorer sleep quality was associated with greater disability via mediating variables of depression, pain, and fatigue; however, results from other studies found no association between sleep quality and disability, though they did not utilize the PSQI.1,4
Although the association between sleep quality and HRQOL/F is well established, more work is needed to understand whether potential mediating variables play a role in this association. Sleep quality is influenced by psychological and physiological factors, and one psychological variable known to influence sleep quality is an individual’s sleep-related beliefs. Those with sleep disturbance often develop distorted beliefs and expectations about the consequences of poor sleep on their well-being and functioning.22,23 Harvey’s Cognitive Model of Insomnia asserts that they may monitor for threats to sleep quality and misperceive actual sleep timing parameters.24,25 Consequently, they may experience autonomic arousal and emotional distress, further disrupting sleep. As people with MS experience higher rates of sleep disturbance than the general population, it seems plausible they may experience a considerable degree of dysfunctional sleep beliefs. Sleep-related beliefs in people with MS have received little attention in the existing literature. The Dysfunctional Beliefs and Attitudes about Sleep Scale-16 (DBAS-16) is a self-report measure that assesses such beliefs.26 Viana and colleagues27 found that dysfunctional sleep beliefs were more prevalent in people with MS and insomnia than in people with MS only, consistent with the general population without MS, but did not examine how these beliefs may have influenced HRQOL/F in people with MS. Schellaert and colleagues28 compared people with MS and insomnia, people with MS only, and people free from neurological disease with and without insomnia on measures of insomnia-associated psychological processes. The researchers found that people with MS and insomnia and people free from neurological disease with insomnia had similar levels of worry and dysfunctional beliefs about poor sleep consequences, which were greater than people with MS only. Although the authors did not examine the association between sleep quality and HRQOL/F, they noted that, on average, people with MS and insomnia had statistically nonsignificant greater disability levels than people with MS only.
The primary aim of the current study was to examine the nature of the association between self-reported sleep quality, dysfunctional sleep beliefs, and HRQOL/F in a sample of people with MS. It was hypothesized that (1) poorer self-reported sleep quality (in line with prior research) and (2) a greater degree of dysfunctional sleep beliefs would be significantly associated with worse MS-related HRQOL/F.
The secondary aim was to determine whether the relationship between self-reported sleep quality, dysfunctional sleep beliefs, and HRQOL/F varied by participant demographic or clinical factors. We hypothesized stronger associations in females due to the higher prevalence of MS and sleep disturbances such as insomnia in women.29,30 Other hypothesized protective factors included younger age, younger age at MS diagnosis, lower/less disability, not progressive MS type, use of disease-modifying therapy (DMT), partnered/married, higher income, and higher education, as these are linked to better HRQOL/F in people with MS.31,32 Race/ethnicity was an exploratory covariate, as there are higher levels of MS in White and Black populations compared with Hispanic and other non-Hispanic groups, though systemic factors (eg, health care access) may confound these interactions.33
The tertiary aim was to explore whether dysfunctional sleep beliefs mediated the association between sleep quality and HRQOL/F. We hypothesized that dysfunctional sleep beliefs would be a significant mediator of the association between sleep quality and HRQOL/F.
Methods
Procedure and Recruitment
This study involved cross-sectional analysis of a data set that combined previously collected data from the Multiple Sclerosis Performance Test (MSPT) with newly collected data on sleep quality and beliefs via the PSQI and DBAS-16. Data collection occurred from March 17 to March 27, 2023, with MyChart messages sent to 952 eligible participants. Survey responses were monitored, and participation was closed upon obtaining 200 unique, complete data sets. Initially, 227 responses were received; however, 27 were excluded due to duplication or incomplete/partial data.
The MSPT, developed at the Cleveland Clinic Mellen Center for Multiple Sclerosis (CC MCMS), objectively measures disease status, progression, and improvement.34 Completed on an electronic tablet during clinic visits, it includes technology enabled neuroperformance tests as well as patient-reported outcome measures (PROMs). The PROMS are the Patient Health Questionnaire-9 [PHQ-9], the Quality of Life in Neurological Disorders (Neuro-QoL), the Patient-Determined Disease Steps (PDDS),35 and Patient-Reported Outcomes Measurement Information System Global Health (PROMIS-GH).
Participants were recruited from the pool of established patients at the CC MCMS. Eligible individuals were at least 18 years old, were diagnosed with MS, had completed the MSPT PROM no more than 3 months prior to completing the sleep questionnaires (MSPT data are part of CC MCMS Clinical Practice Data Registry Institutional Review Board [IRB] #19-1105; IRB of the current study is #22-1294), and were MyChart users. Participants were not recruited based on a prespecified degree of sleep disturbance or diagnosed sleep disorder, as we were interested in capturing the full spectrum of possible sleep disturbance degree in our sample.
Eligible patients were contacted via MyChart message with the study’s purpose, participation requirements, alternatives, risks/benefits, costs, coinvestigator contact information, and a Research Electronic Data Capture (REDCap) link to complete 2 sleep questionnaires. Participants were informed that completing the questionnaires indicated their consent to participate. Each participant received $20 reimbursement. Electronic data were stored in the REDCap database and merged with MSPT data to complete the study data set. Participant demographic/clinical information included medical record number, age, sex, race/ethnicity, marital status, median income by zip code, education level, age at MS diagnosis, MS disease type, PDDS score, and current DMT.
Assessments
Sleep
The PSQI is a 19-item self-report measure assessing 7 components of sleep quality: subjective quality, latency, duration, efficiency, disturbances, use of sleep medication, and daytime dysfunction.36 A global PSQI score ranges from 0 to 21, with higher scores indicating worse sleep quality. Global scores of more than 5 yield a diagnostic sensitivity of 89.6% and specificity of 86.5% for distinguishing between good and poor sleep (κ = 0.75; P < .001). Internal consistency is robust with Cronbach α of 0.83 and test-retest reliability of 0.85 (P < .001) over an average interval of 28.2 days.36 Cronbach α was good at 0.74 in our sample. Jerković et al confirmed the validity and reliability of the PSQI in people with MS, underscoring its utility in this population.37
The DBAS-1626 is a self-report measure assessing sleep beliefs using a Likert-type scale from 0 (strongly disagree) to 10 (strongly agree). A total score is calculated by averaging item scores, and higher scores indicate greater dysfunctional attitudes and beliefs about sleep. It is divided into 4 subscales: consequences of insomnia, worry/helplessness about insomnia, sleep expectations, and sleep medication use. The DBAS-16 demonstrates good reliability, with Cronbach α values of 0.77 for clinical and 0.79 for research samples, and strong test-retest reliability (r = 0.83; P < .001) over a 2-week interval.26 Cronbach α was good at 0.87 in our sample.
Quality of Life
Neuro-QoL is a validated measure of HRQOL/F in individuals with neurological disorders and evaluates 3 domains: physical (eg, fatigue, sleep disturbance), mental (eg, anxiety, depression), and social health (eg, participation in and satisfaction with social roles).38 It has good internal consistency, test-retest reliability, and concurrent and known group validity in people with MS.39 Neuro-QoL scales were collected using computerized adaptive testing, so we cannot report Cronbach α for those scales.
The PROMIS-GH is a 10-item measure assessing general domains of health and functioning and includes 2 subscale scores for physical and mental health.40 Both Neuro-QoL and PROMIS-GH scores are expressed as t scores with a mean of 50 and SD of 10. Higher scores indicate more of the construct being measured (eg, higher t scores on the Neuro-QoL depression scale indicate more depressive symptoms whereas higher scores on the PROMIS-GH physical health scale indicate better physical health). In our sample, Cronbach α was good for PROMIS-GH mental (0.87) and physical (0.81) health.
Statistical Analysis
Sample size was calculated a priori, as independent variables, covariates, and interaction terms required estimation of approximately 11 to 13 model parameters. Thus, a total of 15 patients per estimated parameter was deemed sufficient for accurate estimation of each parameter.
Descriptive statistics summarizing patient and clinical characteristics were calculated for the sample. Pearson correlations between PSQI and HRQOL/F PROM (Neuro-QoL, PROMIS-GH, PHQ-9) and between DBAS-16 and HRQOL/F PROM were also computed.
For the primary aim, separate multivariable linear regression models were fit where each model included one of the HRQOL/F PROM scores as the dependent variable. For each HRQOL/F PROM, 2 models were fit: one with PSQI score and one with DBAS-16 score as independent variables. Assumptions for linear regression were met. In all models, the following covariates were included: age, sex, race (White vs Black, Asian, American Indian/Alaska Native, other), marital status (married/partnered vs not married/partnered), median income by zip code, education level (college/graduate/professional vs some high school/high school/General Educational Development test/some college), age at MS diagnosis, MS disease type (progressive [secondary progressive, primary progressive, progressive relapsing] vs not progressive [relapsing remitting, 1 relapse or episode of vision problems, not diagnosed with MS or CIS]), PDDS (no identified gait disability, mild/moderate gait disability, cane/bilateral support/wheelchair), and currently taking DMT (yes vs no).
Our secondary aim was to determine whether the relationship between sleep quality, dysfunctional sleep beliefs, and HRQOL/F differed based on demographic/clinical covariates. Two-way interactions between PSQI score, DBAS-16 score, and each demographic/clinical factor were added in separate models one at a time. Statistical significance of the interaction term indicated that the PSQI or DBAS-16 differentially affected the HRQOL/F PROM score for that demographic/clinical variable.
For the tertiary aim, we performed mediation analysis where each HRQOL/F PROM was the dependent variable, PSQI score was the independent variable, and DBAS-16 score was the mediator. We used bootstrap methods to estimate and test for indirect, direct, and total effects. We used 1000 bootstrap samples, and 95% CIs were constructed using the bootstrap percentile method.
Computations were performed in R, version 4.3.1. All tests were 2-sided, and statistical significance was established at P < .05. As we examined multiple HRQOL/F PROMs, we accounted for multiple testing using the Holm method.
Results
Of the 200 participants, 197 had complete DBAS-16 data and 180 had complete PSQI data (133 of 180 [73.9%] had a PSQI total score > 5). Table 1 presents descriptive statistics detailing participant demographic/clinical characteristics. Table S1 displays mean scores for PROMIS-GH and Neuro-QoL scale t scores and Pearson correlations between these scales and the PSQI and DBAS-16. Both PSQI and DBAS-16 were most strongly correlated with Neuro-QoL sleep disturbance (r = 0.540 and r = 0.613, respectively) and most weakly correlated with Neuro-QoL lower extremity (r = –0.110 and r = –0.005, respectively). After sleep disturbance, PSQI was most strongly correlated with Neuro-QoL depression (r = 0.411) and DBAS-16 was most strongly correlated with Neuro-QoL fatigue (r = 0.448). The correlation between PSQI and DBAS-16 scores was 0.64.
Table 2 displays multivariable linear regression model results for each PROMIS-GH or Neuro-QoL t score where PSQI or DBAS-16 was the independent variable. After adjusting for covariates and correcting for multiple testing, higher PSQI scores (poorer sleep quality) were associated with lower t scores for PROMIS-GH physical and mental health and the Neuro-QoL scales of cognitive function, satisfaction with social roles, depression, anxiety, fatigue, and sleep disturbance. Higher DBAS-16 scores (greater degree of dysfunctional sleep beliefs) were associated with worse t scores on these scales and Neuro-QoL positive affect/well-being.
Table S2 shows results of linear regression models that included interactions between PSQI score and demographic variables (only results of significant interactions shown). The interaction between PSQI score and education level was significant in models for Neuro-QoL satisfaction with social roles and upper extremity function (see Figure S1). As PSQI score increased, t scores for both Neuro-QoL scales decreased (worsened), particularly for patients with less education (some college or less) compared with patients with more education (college degree or more).
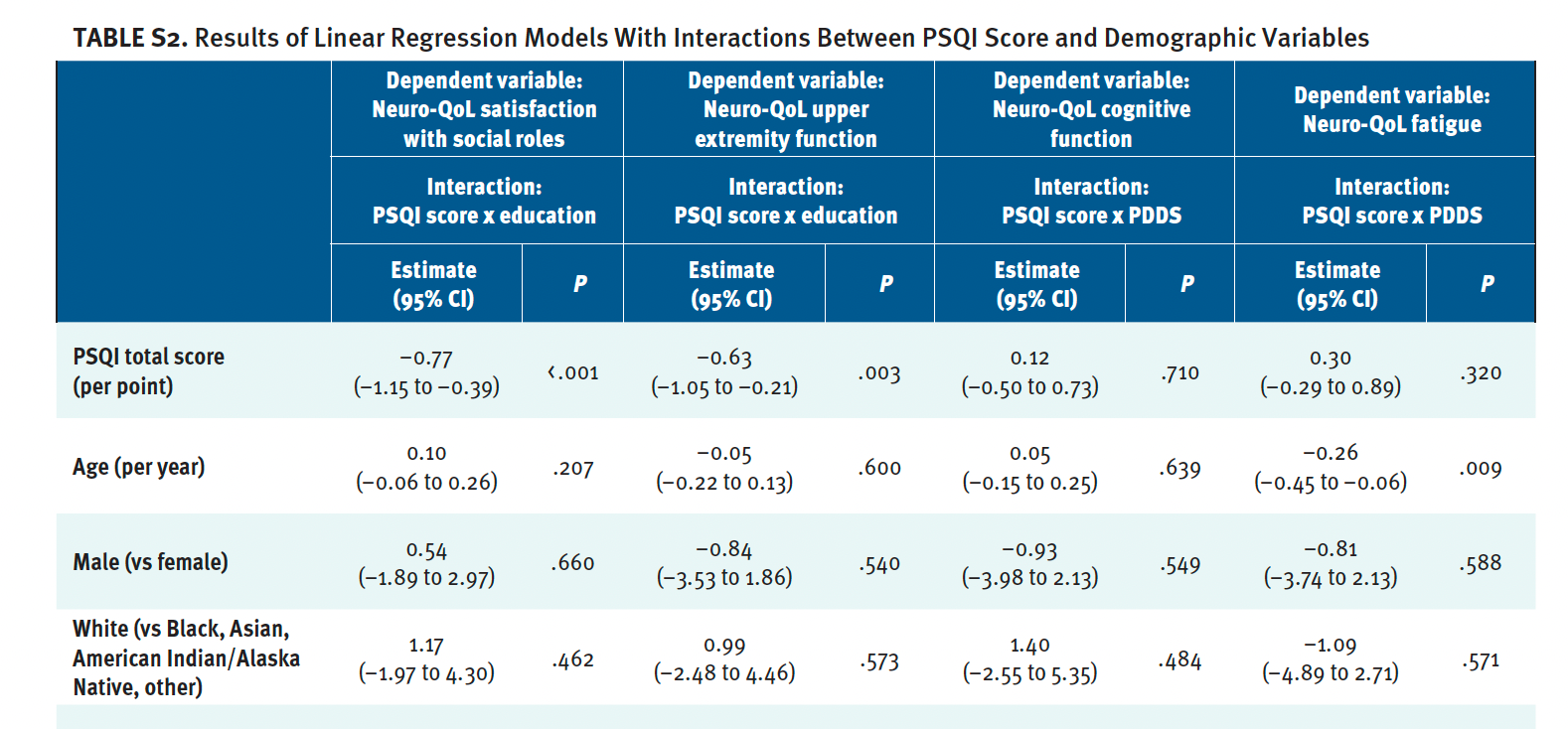
The interaction between PSQI score and PDDS score was significant in the models for Neuro-QoL cognitive function and fatigue (see Figure S2). As PSQI score increased, Neuro-QoL cognitive function t scores slightly increased (improved) for patients with no identified PDDS gait disability but decreased (worsened) for patients with mild/moderate gait disability and patients who used a cane, bilateral support, or wheelchair/scooter. For all 3 PDDS groups, increased PSQI score was associated with higher (worse) Neuro-QoL fatigue t scores but the association was strongest for patients who used a cane, bilateral support, or wheelchair/scooter.
Table S3 shows results of linear regression models that included interactions between DBAS-16 total score and demographic variables (only results of significant interactions shown). The interaction between DBAS-16 score and PDDS score was significant in the models for PROMIS-GH physical health and Neuro-QoL positive affect (see Figure S3). As DBAS-16 score increased, PROMIS-GH t score decreased (worsened), but the association was weaker for patients with mild/moderate gait disability than either patients with no identified PDDS gait disability or patients who used a cane, bilateral support, or wheelchair/scooter. As DBAS-16 score increased, Neuro-QoL positive affect/well-being score slightly increased for patients with no identified PDDS gait disability but decreased for patients with mild/moderate gait disability or patients who used a cane, bilateral support, or wheelchair/scooter.
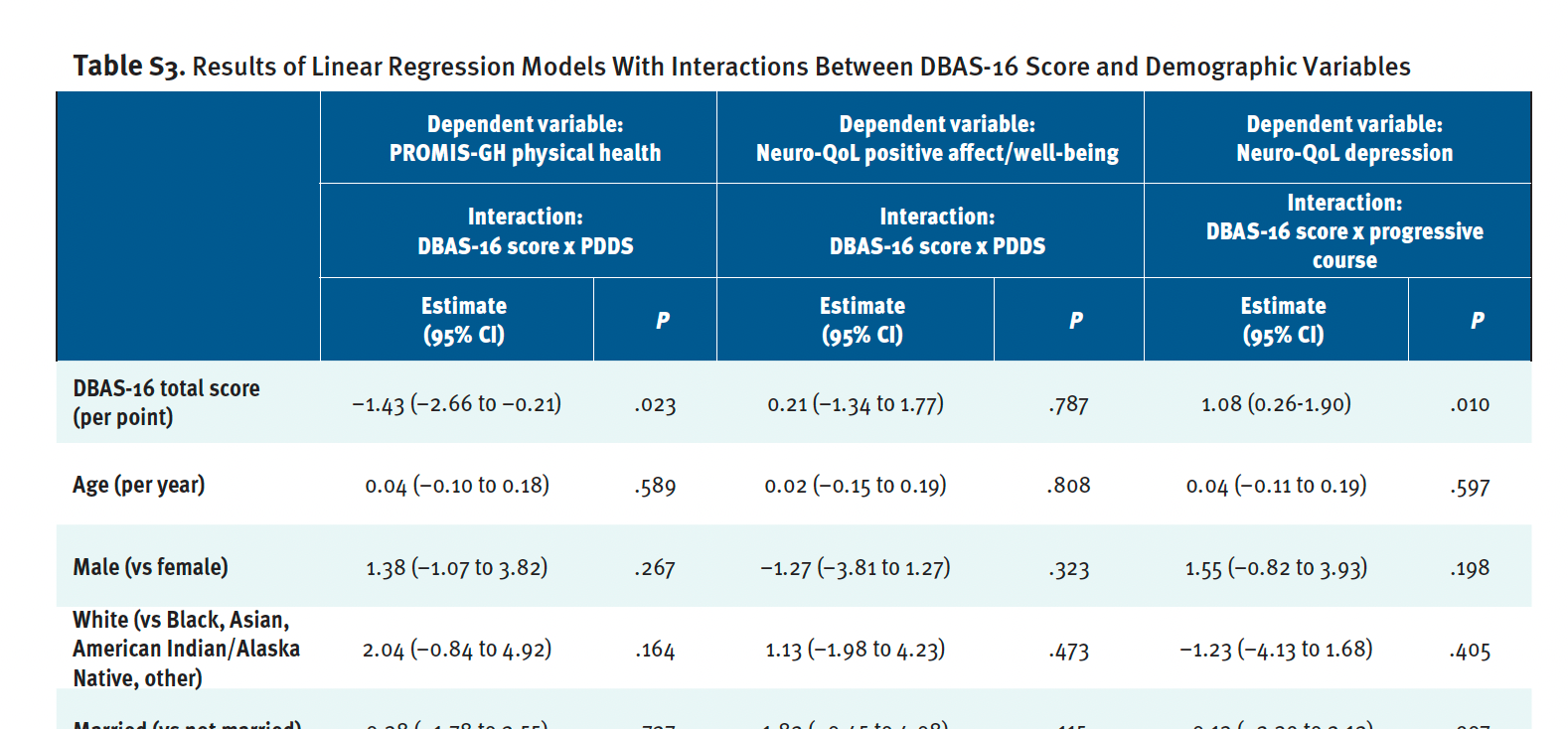
Finally, the interaction between DBAS-16 score and progressive MS type was significant in the model for Neuro-QoL depression (see Figure S4). As DBAS-16 score increased, Neuro-QoL depression t scores increased (worsened), but the association was stronger in patients who had progressive MS than those with not progressive phenotypes.
Table S4 shows results of the mediation analyses. There was a significant mediating effect of DBAS-16 on the relationship between PSQI and the PROMIS-GH mental health and each of the Neuro-QoL cognitive function, ability to participate in social roles, depression, anxiety, stigma, fatigue, and sleep disturbance scales (all indirect effect P values < .05).
Discussion
This study aimed to contribute further evidence of the association between sleep quality and HRQOL/F in people with MS while examining the mediating role of dysfunctional sleep beliefs and assessing whether associations varied by demographic/clinical factors. To our knowledge, this is the first study to explore the role of dysfunctional sleep beliefs in HRQOL/F among people with MS.
Results demonstrated that worse self-reported sleep quality and greater dysfunctional sleep beliefs were each linked to poorer outcomes in the PROMIS-GH scales of mental health and physical health and the Neuro-QoL scales of cognitive function, satisfaction with social roles, fatigue, sleep disturbance, and positive affect/well-being. Significant interactions were revealed between PSQI and education on satisfaction with social roles and upper extremity function, between PSQI and PDDS on cognitive function and fatigue, between DBAS-16 and PDDS on physical health and positive affect/well-being, and between DBAS-16 and progressive MS on depression. Perhaps most interestingly, dysfunctional sleep beliefs significantly mediated the relationship between PSQI and mental health, cognitive function, social role participation, depression, anxiety, stigma, fatigue, and sleep disturbance.
Our findings align with prior research on the association between sleep quality and HRQOL/F as well as the existing literature examining dysfunctional sleep beliefs in chronic conditions. Schellaert et al found that people with MS and comorbid insomnia had significantly higher consequences and worry/helplessness subscale scores on the DBAS-16 than people with MS alone but no significant difference compared with people with insomnia and without MS.28 Although our study did not include people with no neurologic disease, it contributes further data that dysfunctional sleep beliefs common in the general population also occur in people with MS. In considering studies involving other chronic conditions, results from one study found that patients with fibromyalgia exhibit greater dysfunctional sleep beliefs than controls and results from another found that patients with spinal pain show positive correlations between such beliefs and PROMIS pain interference.41,42 Evidence of this association in those with comorbid chronic pain and insomnia led to development of the Pain-Related Beliefs and Attitudes about Sleep scale, suggesting potential for an MS-specific version.43 In Parkinson disease, greater dysfunctional sleep beliefs are linked to increased depressive symptoms, with findings from one study demonstrating that after controlling for depression, the relationship between presleep cognitive arousal and insomnia was mediated by sleep-related safety behaviors and dysfunctional sleep beliefs.44,45
In addition to the main findings, some results involving the influence of demographic/clinical factors on sleep quality, sleep beliefs, and HRQOL/F were unexpected, posing interpretive challenges. Poorer sleep quality was associated with less satisfaction with social roles and poorer upper extremity functioning, particularly among those with less education. This suggests that higher education may offer protective effects, possibly via access to a broader range of social roles (thus, increased chance of satisfaction with those roles) or access to better resources and/or services to support upper extremity functioning. Moreover, interaction terms showed worse sleep quality was associated with poorer cognitive functioning for patients with mild/moderate gait disability or mobility aid use but also slight (nonsignificant) improvement in patients with no PDDS identified gait disability, possibly indicating that better functioning/mobility may mitigate the impact of poor sleep on cognitive functioning. Finally, greater dysfunctional sleep beliefs were associated with worse physical health, especially for patients with no PDDS identified gait disability and those using mobility aids compared with those with mild/moderate gait disability. These outcomes suggest that dysfunctional sleep beliefs may have a more pronounced effect at the extremes of the functioning spectrum, though underlying factors driving these associations remain unclear. Of note, collapsing the PDDS scale scores from the original 9 categories to 3 categories of disability may have obscured results by artificially reducing variability in disability level. These nuanced results underscore the complex interplay between sleep quality, sleep beliefs, and HRQOL/F in people with MS, warranting further research to clarify underlying mechanisms.
This study has several limitations. First, the sample was predominantly White, non-Hispanic, middle-aged, female participants with college degrees, middle-class income, and no PDDS identified gait disability, limiting generalizability and the ability to test some hypothesized covariate effects. Future studies should aim for broader representation. Online data collection may have reduced heterogeneity in our sample, as participation required electronic device access and internet service, factors possibly related to income level or degree of disability. Second, this study utilized self-report measures of sleep and HRQOL/F, and future studies might incorporate objective measures such as actigraphy or polysomnography for more comprehensive assessment of sleep quality. Third, averaging DBAS-16 scores into a global score may have limited insight into specific types of dysfunctional sleep beliefs experienced by participants, which should be explored in future studies. Fourth, the cross-sectional study design and difference in timing between MSPT and sleep questionnaire completion between participants limited causal inference and information on temporal relationships between sleep quality and HRQOL/F. Findings from longitudinal studies could reveal how these relationships evolve over time or across the MS disease course (eg, examining changes in sleep quality and beliefs during MS relapse). Future studies might consider whether sleep problems and dysfunctional beliefs preceded MS diagnosis and were exacerbated by MS symptoms and the possibility of a bidirectional association between sleep quality and HRQOL/F. Finally, this study did not assess specific causes of sleep disturbance or comorbid sleep disorders such as sleep apnea or restless legs syndrome, which are common in people with MS.46-48 Future research should account for these factors to explore physiological mechanisms underlying sleep quality, sleep beliefs, and HRQOL/F.
Conclusions
The results of this study have important clinical implications. Routine assessment for physical causes of sleep-related concerns to improve HRQOL/F in people with MS, including MS symptoms that may manifest as sleep disturbance (eg, spasticity, nocturia, pain), is always warranted. Our results suggest an additional avenue to improving HRQOL/F via addressing dysfunctional sleep beliefs. Cognitive behavioral therapy for insomnia (CBT-I) is an intervention well equipped to address such beliefs via cognitive restructuring. CBT-I has been shown to be feasible and efficacious in people with MS, improving insomnia severity, sleep quality, and comorbid fatigue, depression, anxiety, and sleep self-efficacy.49-51 In considering how MS diagnosis may influence dysfunctional sleep beliefs, it is plausible that patients’ knowledge of increased sleep disturbance risk associated with MS heightens these beliefs, but it is more likely via experiential learning. People with MS are likely to experience a vicious cycle in which they develop sleep disturbance, triggering unhelpful thoughts/beliefs about sleep, which increases stress/arousal and perpetuates sleep disturbance. Specific beliefs may include fears that poor sleep will trigger relapses, worsen neurological symptoms, exacerbate MS-related fatigue, or require sleep aids to counteract the influence of MS symptoms on sleep. Future research is warranted to explore the nature of MS-specific dysfunctional sleep beliefs, which could be targeted via cognitive restructuring.
Understanding the relationship between sleep quality, dysfunctional sleep beliefs, and HRQOL/F is crucial to inform clinical initiatives aimed at enhancing sleep and ultimately HRQOL/F in people with MS. Moreover, identifying the demographic/clinical factors that influence this relationship can assist clinicians in providing more personalized, patient-centered care.

















